The Effect of Simvastatin in Penile Erection
Abstract
The aim of the study is to evaluate the effect of simvastatin in erectile dysfunction (ED) secondary to endothelial dysfunction. This study is a double-blind, randomized, placebo-controlled, clinical trial in patients with ED and endothelial dysfunction. Patients were randomized to receive 20 mg simvastatin (n=21) or placebo (n=20) daily for 6 months and subsequently 10 mg of vardenafil on demand for 4 weeks. Serum cholesterol, hormone profile, ultrasensitive C-reactive protein, the International Index of Erectile Dysfunction (IIEF) and the ED Index of Treatment Satisfaction were evaluated. There was a significant reduction in serum cholesterol in the treatment group. The hormonal profile remained unaltered. There was no difference in the IIEF between the groups at follow-up, although, at the beginning, 26% of the patients of both groups presented with mild ED and 74% with moderate-to-severe ED; at the end of the 7th month, all patients from the simvastatin group progressed to mild ED, compared with only 83% in the placebo group. There was no statistically significant difference in penile erection after intake of simvastatin or placebo. This study does not support the use of simvastatin as erectogenic medication. Further studies are necessary to verify if simvastatin has any beneficial effect on ED.
Introduction
Recent evidence has suggested that erectile dysfunction (ED) is strongly correlated with other correlates of endothelial dysfunction,[1,2] such as coronary atherosclerotic disease. Obesity, dyslipidemia, smoking, sedentarism, systemic arterial hypertension and diabetes mellitus are all risk factors for the development of vascular dysfunction and atherosclerosis, often present in men with ED.[3] According to Thompson et al.,[2] in some situations ED can even precede the onset of clinically manifested vascular disease. Endothelial dysfunction contributes to ED mainly through a change in nitric oxide (NO) metabolism. High levels of ultrasensitive C-reactive protein (usCRP) indicate the presence of endothelial dysfunction.[4–6]
Statins, primarily used for the treatment of hypercholesterolemia, have pleiotropic effects such as reducing the risk of cardiovascular events. This occurs through the upregulation of the systemic vascular endothelial activity and the NO metabolism.[7,8] Current knowledge about the physiopathology of ED has led to the development of clinical trials to assess the role of statins in men with ED. However, published data about the interaction of ED and statins are sparse and conflicting. Earlier studies suggested that ED could develop as a consequence of statin use,[9–12] whereas others did not demonstrate any such association.[8,13–15] By contrast, more recent studies have suggested the possibility that statins may improve ED in men with endothelial dysfunction.[16–26] Therefore, research evaluating the potential benefit of statin use in the treatment of men with ED determined by endothelial dysfunction is needed, especially regarding the pleiotropic effects of this class of medication.
The aim of this study is to evaluate the effects of simvastatin in ED secondary to endothelial dysfunction, using the International Index of Erectile Dysfunction (IIEF) and the ED Index of Treatment Satisfaction as instruments.
We performed a double-blind, randomized, placebo-controlled, prospective clinical trial to evaluate the effect of simvastatin (20 mg per day) on the erectile function of patients with ED secondary to endothelial dysfunction. The study was performed in patients selected at an andrology outpatient clinic of the Urology Service of Santa Casa de Porto Alegre, from January 2006 to December 2007. The study protocol was approved by the committee on ethics in research of Santa Casa de Porto Alegre and by the ethics committee of the Instituto de Cardiologia and is registered at ClinicalTrials.gov under identification number NCT00947323 (Simvastatin Treatment for Erectile Dysfunction—STED TRIAL). The calculated sample size (34 patients) was estimated based on a P α<0.05 and on a P β<0.2, to ascertain an expected 40% difference in the IIEF score between treatment groups.
We included patients with ED based on clinical history and an IIEF-5 score under 22, aged 35–75 years with levels of usCRP ≥1.1 mg l−1 and no other medical indication or contraindication for the use of statins. We excluded patients with a history of acute myocardial infarction or stroke, diabetes mellitus, Peyronie’s disease, hypogonadism, hyperprolactinemia or any active inflammatory or infectious disease. We also excluded from the study alcohol abusers and patients who underwent radical prostatectomy, pelvic surgery or radiation therapy, as well as those who had used any phosphodiesterase-5 inhibitor (iPDE5) during the 6 months before enrollment.
After being informed about the details of the study orally and in printed matter, those who agreed to participate signed a free and informed consent form. Patients were randomized to receive 20 mg simvastatin or placebo orally in a single nightly capsule for 6 months, by randomly drawing lots previously defined by a non-participant of the study. The medication was administered in a double-blinded manner. The patients were not allowed to take any erectogenic drug during the 6 months of treatment with simvastatin or placebo, in order to avoid any influence of erectogenic medication or confusion bias during the evaluation of the response to the treatment with simvastatin. In addition, all patients were oriented to intake 10 mg of vardenafilat 1 h before sexual intercourse, twice a week, for 4 weeks, after the initial 6 months of daily simvastatin or placebo. A pharmacist alien to the study was responsible for both the release and the control of the medications. The substance contained in the blinded medication was only disclosed after the final statistical analysis of the data.
The patients’ erectile function was evaluated by validated questionnaires (IIEF and ED Index of Treatment Satisfaction) in native language—Portuguese. The severity of ED was evaluated by IIEF erection function domain (normal ≥26, mild ED=22–25, mild-to-moderate ED=17–21, moderate ED=11–16 and severe ED≤10). All blood tests and questionnaires were applied at enrollment in the study and repeated every 2 months, for 6 months, and after 1 month of on-demand use of 10 mg vardenafil. The questionnaires were applied at two distinct moments, after simvastatin alone and after vardenafil, in order to evaluate the effect of the previous intake of simvastatin on the vardenafil response. At the end of the study, the patients answered an additional question about overall efficacy: ‘Did the treatment you underwent improve your erections?’ The primary end point was to evaluate the penile erection after the use of simvastatin; the secondary one was to evaluate the response to vardenafil with previous use of simvastatin, to check the effect of iPDE5 after endothelial dysfunction correction with statin.
Once the patients had completed the 7 months of the study, they remained under follow-up at the andrology clinic, receiving standard treatment for ED. The study was monitored by an external committee, unconnected to the research group. Any clinical event reported by the patient after the beginning of the medication was noted and classified as an adverse effect whenever applicable.
Results were analyzed using the Statistical Package for the Social Sciences (SPSS, DMSS, São Paulo, SP, Brazil), version 17.0. Analysis was performed by intention to treat, without revealing which medication the patient had received. The statistical tests applied were Student’s t-test, Mann–Whitney test, Wilcoxon Signed Rank, χ 2 and analysis of variance for repeated measures.
Results
Of the 263 patients initially evaluated, 43 who fulfilled the inclusion/exclusion criteria and agreed to sign the letter of informed consent were randomly assigned to the simvastatin or placebo groups. Of these, two patients were lost during follow-up, one from each group, having dropped out of the study without an apparent cause. In all, 41 patients were followed until the end of the study, and constitute our final sample.
The baseline characteristics of the randomized patients are shown in Table 1, which demonstrates that there is no difference between the groups after randomization. Their mean age was 57.5±8.3 years, 89% of them were Caucasian.
Table 2 shows that, although non-significant, the usCRP levels reduced from 3.8 to 2.5 mg dl−1 in the simvastatin group (reduction of 32%), whereas in the placebo group it reduced from 2.8 to 2.6 mg dl−1 (reduction of 8.3%).
Table 2 also shows that there was a significant reduction in the total cholesterol, LDL and triglyceride levels in the simvastatin group. After 6 months of treatment, there was no difference between the groups in terms of testosterone, luteinizing hormone, follicle-stimulating hormone, prolactin and estradiol.
The body mass index did not vary significantly, ranging from 28.44 to 28.41 in the placebo group and from 27.40 to 27.68 in the simvastatin group (P=0.556).
Erectile function evidenced no statistical significant difference neither after 6 months of treatment with simvastatin (20 mg) or placebo nor after vardenafil, according to IIEF and ED Index of Treatment Satisfaction, as seen in Table 3.
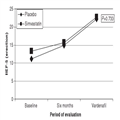
Figure 1 shows the evolution of IIEF Erection over time in both groups.
|
Figure 1.
International Index of Erectile Dysfunction according to the group Simvastatin or Placebo and the period of evaluation. |
Figure 1.
Table 4 shows that, although there was no statistically significant difference between the groups at the beginning of the study, 73.6% and 73.7% of the patients in the simvastatin and placebo group, respectively, had moderate-to-severe ED. After 6 months of treatment with simvastatin, 100% of the simvastatin-treated group had evolved to mild ED compared with 83.3% of the placebo group.
As to adverse effects, two patients in the placebo group reported events (one case of insomnia and the other of dyspepsia). A patient in the simvastatin group mentioned insomnia.
Discussion
The results of this study show that simvastatin did not determine a statistically significant difference of erectile function compared with the placebo even after the vardenafil intake. We can see, however, that at the beginning of the study, 26% of the patients in both groups were classified as having mild ED and approximately 74% as having moderate-to-severe ED. At the end of 7 months, all the patients treated with simvastatin had evolved to mild ED, whereas in the placebo group, 83% had evolved to this degree of dysfunction, though this was not statistically significant.
There was no statistically significant difference between the groups as regards reduction in the usCRP levels. However, the group treated with simvastatin 20 mg presented a 32% reduction, whereas the placebo group presented a reduction of only 8%. It is important to consider that the reduction in usCPR may represent the modulation of the endothelial function induced by simvastatin, although there was no statistical significant difference in terms of ED between the groups, probably because of the small number of the patients.
As to changes in the lipid profile, however, it became very clear that simvastatin at the 20 mg dose significantly reduced the total cholesterol, low-density lipoprotein and triglyceride levels.
As to ED, our results are opposite to those of previous studies, which suggested that the statins may trigger ED. Actually, in those studies, most of which were case reports or small historical series, there was a selection of very ill patients with many associated morbidities, confounding a careful evaluation of ED.[9–12] In other publications, the relationship between the use of statins and the development of ED has been considered an undefined topic.[8,13–15] In our study, with patients without clinical manifestation of vascular disease and no associated comorbidities, simvastatin proved safe and effective to reduce the cholesterol levels. It did not lead to any improvement in ED or determine any reduction in the levels of testosterone and the other hormones evaluated. As to maintaining the hormone levels, our results find physiological basis in the studies by Dobs et al.,[15] in which, according to the authors, patients treated with pravastatin 40 mg, simvastatin 20 and 40 mg or placebo did not present differences in the measures of total and free testosterone.
ED is currently much discussed from the perspective of endothelial dysfunction. It is known that in about 50% of men with ED above the age of 50 years, there is an association with vascular disease. It is postulated that endothelial dysfunction occurs in the presence of risk factors, and its consequences are felt throughout the vascular endothelial bed, including the penis. In this dysfunction, there is a change in the NO metabolism in the penile corpus cavernosum, determining a state of arteriolar vasoconstriction, with a reduction of the blood inflow and consequent deficiency in penile erection. In this sense, the use of statins and the acquisition of their pleiotropic effects, especially on the vascular endothelial bed, may be relevant for the management of patients with ED and endothelial dysfunction.
Our study looked at the use of statins in potentially healthy patients, in which the main indication of endothelial dysfunction was elevated levels of usCRP. The JUPITER study showed the safety and efficacy of the use of statins in primary prevention, selecting patients according to the usCRP levels.[27] Some authors showed that the treatment of dyslipidemia with statins can determine the improvement of ED in selected patients.[16,18,19,21–23,26] The explanations of how statins could improve ED are based mainly on the pleiotropic effects of this class of medication, that is, effects that go beyond simply lowering cholesterol levels.[7,8] Outstanding among these effects are the stabilization and posterior reduction of the atherosclerotic plaque, improved endothelial function and regulation of NO production, with consequent arteriolar vasodilation and diminished inflammatory activity, oxidative stress and thrombotic events.
Another point discussed in the literature is that the use of statins may modulate the response to iPDE5, so that patients who are initially non-responsive to iPDE5 benefit from these medications, especially through the mechanism mediated by NO.[18,19,21,22,26] Our results do not support this supposed improved response to iPDE5 after the use of simvastatin, although the studies that signal this endothelial modulation used atorvastatin.[16,18,19,22,23,26]
It may be that the improved response to iPDE5 reported in these studies is related to the potency of the statin s−1 used. This is suggested by three randomized clinical trials using atorvastatin (from 40 to 80 mg daily), that showed a significant improvement in ED compared with the placebo[19,21,26] after less than 6 months using the medication. These studies suggest that the length of the treatment with simvastatin in our patients (6 months) should have been sufficient to detect changes in ED. A study that evaluated 50 men without previous ED and without dyslipidemia submitted to radical prostatectomy, were randomized to receive sildenafil on demand or sildenafil on demand plus atorvastatin 10 mg daily for 90 days, after discharge from hospital. The patients who received atorvastatin presented a significant increase in IIEF-5 (13.5 against 10.6 in the patients who did not receive atorvastatin P=0.003), suggesting that the use of atorvastatin after radical prostatectomy with nerve preservation may contribute to a better recovery of erectile function. However, in this study there was no placebo group, which may limit the interpretation of the results.
There are several limitations to be considered in this study, including the small size sample, the limited dose ranges of the simvastatin-treated group and the lack of a physiological study of penile vascular function, such as Doppler ultrasound measures that could assess the endothelial function by flow-mediated dilation (such as endo-PAT 2000, Itamar Medical Inc., Framingham, MA, USA). Further limitations would be that the most significant benefits for ED with simvastatin may be reached at higher doses, such as 40 mg, or higher, and that the difference between groups may be <40%, which determines the need for a greater number of patients for evaluation. It is possible that with the simvastatin dose administered, we did not achieve a sufficiently robust improvement in endothelial function to provoke modification in the ED. These questions could be better answered with clinical tests evaluating simvastatin at larger doses or using a more potent statin.
The results of this study demonstrated that simvastatin did not determine a statistically significant improvement of erectile function compared with the placebo and do not support the use of simvastatin as erectogenic medication. It seems unlikely that simvastatin determines ED. On the other hand, the evolution observed in the severity of ED suggests that simvastatin may help improve the erectile function of patients with ED and endothelial dysfunction concomitantly with administration of iPDE5. Additional studies with more patients will be necessary to detect smaller differences in the responses obtained with simvastatin in relation to the placebo.